Sunday, December 23, 2007
Christmas decorations for chemists
Friday, December 21, 2007
+PLoS+
 Some time ago when I was ranting about papers, authors and so on I promised a post on open access journals, more specifically PLoS: Public Library of Science. I'll leave the Open Access issue for another day, suffice to say it's something I like very much. Anyway, the PLoS journals have several neat features:
Some time ago when I was ranting about papers, authors and so on I promised a post on open access journals, more specifically PLoS: Public Library of Science. I'll leave the Open Access issue for another day, suffice to say it's something I like very much. Anyway, the PLoS journals have several neat features: So why am I not publishing all my papers in open access journals. Well chemistry still hasn't caught up with things in this area. There are things happening but compared to what's going on in biology we have a long way to go. However, this is the way of the future and the journals know it so they will have to adapt. Recently, a bill that required all NIH funded research to be published in open access journals was vetoed at a late stage by President Bush so things are certainly bubbling away in this area.
So why am I not publishing all my papers in open access journals. Well chemistry still hasn't caught up with things in this area. There are things happening but compared to what's going on in biology we have a long way to go. However, this is the way of the future and the journals know it so they will have to adapt. Recently, a bill that required all NIH funded research to be published in open access journals was vetoed at a late stage by President Bush so things are certainly bubbling away in this area. 
Merry Christmas and Happy New Year people. No more posting this year. See you in 2008 and thanks for all the interesting and very encouraging emails. D!
Saturday, December 08, 2007
Colourful Chemistry
 I'm doing lots of new chemistry in my new job which is great. Since I started my career as an organic chemist more or less everything has been supposed to be colourless or white.
I'm doing lots of new chemistry in my new job which is great. Since I started my career as an organic chemist more or less everything has been supposed to be colourless or white.What makes the chemistry I'm doing now even greater is that it has colour whilst still being organic chemistry.
And how about this product from another reaction? Initially I assumed that the product was impure but what do you know it's supposed to like like this.
In your face inorganic chemists! Can anyone guess what I made? It is a classic reaction taught in first year university organic chemistry. It's nice to see the chemistry I have been teaching undergraduates is useful and actually works in the lab. D!

Wednesday, December 05, 2007
Compound Characterisation in Industry
 As I have mentioned previously I'm back working in industry/biotech. One of my colleagues asked me an interesting question today:
As I have mentioned previously I'm back working in industry/biotech. One of my colleagues asked me an interesting question today:Wednesday, November 21, 2007
Low Vacuum Manifold
Tuesday, November 20, 2007
Monday, November 12, 2007
Grease

Saturday, November 10, 2007
Curly Arrow update
 Apologies for the infrequent posting. I'm very happy to see that people keep coming to the blog despite this. So what's my excuse this time? Well I just started a new job. I have left academia (for now) and I'm working in a medium size biotech company doing some traditional medicinal chemistry to cure the world etc. However, I have been made an adjunct research fellow at the university and continue to supervise some students so I still have a foot inside the academia door. Also I have become a regular writer for the chemistry magazine published by the Danish Chemical Society which is quite exiting. Unfortunately this only gets published in danish so it won't be of interest to most of you guys. What's even more cool is that they have agreed to set up a blog on the magazines web page where the articles will also be published.
Apologies for the infrequent posting. I'm very happy to see that people keep coming to the blog despite this. So what's my excuse this time? Well I just started a new job. I have left academia (for now) and I'm working in a medium size biotech company doing some traditional medicinal chemistry to cure the world etc. However, I have been made an adjunct research fellow at the university and continue to supervise some students so I still have a foot inside the academia door. Also I have become a regular writer for the chemistry magazine published by the Danish Chemical Society which is quite exiting. Unfortunately this only gets published in danish so it won't be of interest to most of you guys. What's even more cool is that they have agreed to set up a blog on the magazines web page where the articles will also be published.I'd like to thank people for the many emails I get with interesting questions and requests for paper reprints. Thanks for taking an interest. Curly Arrow has now been running for 1 year and seems to be a fairly popular blog (see some stats below). I've even reached the point where people are offering me money to place adds on the blog (You have to pay a lot more guys if you even want me to reply to those emails) and I have been blessed with lots of spam comments linking to porn sites (the pinnacle of my career). I almost clicked on cucumber sex to see what cucumber variety would be employed. D!
Monday, October 08, 2007
Authors - alphabetical vs. by contribution
 After my first post on Authors and who goes on papers I have received quite a few emails from people and had some interesting discussions with some of my colleagues. It seems that everyone has a good story on this topic. Something that I've noticed can give rise to some heated discussions is whether authors should be listed alphabetically or according to how much they have contributed, i.e. the first author did most. From what I've gathered so far the alphabetical system seems to be a chemistry thing. When I mention this concept to people in biology/biochemistry they are outraged. In their area you really need to be the first or second author for the paper to carry any serious weight on your publication list. After thinking a bit about all this I have reached two conclusions that may or may not be right:
After my first post on Authors and who goes on papers I have received quite a few emails from people and had some interesting discussions with some of my colleagues. It seems that everyone has a good story on this topic. Something that I've noticed can give rise to some heated discussions is whether authors should be listed alphabetically or according to how much they have contributed, i.e. the first author did most. From what I've gathered so far the alphabetical system seems to be a chemistry thing. When I mention this concept to people in biology/biochemistry they are outraged. In their area you really need to be the first or second author for the paper to carry any serious weight on your publication list. After thinking a bit about all this I have reached two conclusions that may or may not be right:Thursday, September 27, 2007
NMR tube cleaner
 I have a lot of NMR tubes and as a result I don't clean them very often and when I eventually decide to do something about it I'm faced with something like 30 dirty tubes. Now firstly if you work this way you have to soak the NMR tubes as soon as you are done with them. If you just let them evaporate to dryness you are in trouble. I normally just empty the tube and fill it to the lip with acetone and cap it. This way it wont dry out for months.
I have a lot of NMR tubes and as a result I don't clean them very often and when I eventually decide to do something about it I'm faced with something like 30 dirty tubes. Now firstly if you work this way you have to soak the NMR tubes as soon as you are done with them. If you just let them evaporate to dryness you are in trouble. I normally just empty the tube and fill it to the lip with acetone and cap it. This way it wont dry out for months.Friday, September 21, 2007
Authors - who goes on the paper and why?
 Yes I know! It's pretty quiet around here. My Internet connection at home is dead. I'm on the phone with my Internet provider every day to resolve this issue but it is a slow process.....So I guess a bit of blogging from work is required (Sorry boss. I'll run that column in 15 minutes). I'm writing papers at the moment and this always brings up the issue of authors. Who's on and who's off? Over the years I've experienced some pretty disturbing things in this category:
Yes I know! It's pretty quiet around here. My Internet connection at home is dead. I'm on the phone with my Internet provider every day to resolve this issue but it is a slow process.....So I guess a bit of blogging from work is required (Sorry boss. I'll run that column in 15 minutes). I'm writing papers at the moment and this always brings up the issue of authors. Who's on and who's off? Over the years I've experienced some pretty disturbing things in this category:Wednesday, August 22, 2007
Pay Rise Cake
Banana and Chocolate Cake aka Pay Rise Cake [Serves many, 20+]
Ingredients
Flour (500 g)
Sugar (500 g)
Quick oats (50 g)
Butter (300 g)
5 x 50 g eggs
8 ripe bananas (ca 700-800 g when peeled)
Skim milk (150 ml)
Vanilla sugar (5 tsp)
Baking powder (5 tsp)
Salt (3 tsp)
Dark chocolate (300 g, Don't be a cheap skate and use good stuff. For example Lindt 70%)
(1) Melt the butter - do not reflux
(2) Beat the sugar with the melted butter
(3) Add the eggs (not the shells) and beat
(4) Add milk and bananas (mash the bananas first) and beat
(5) Add flour, quick oats, vanilla sugar, baking powder and salt and beat
(6) Chop some of the chocolate (200 g) and mix it with the dough (don't beat it at this stage)
(7) Transfer the dough to a large baking tray (eg. 30 x 30 x 5 cm) and bake in the centre of the oven at 180 oC for 40-45 minutes. When the dough doesn't stick to a metal object it is finished (you basically insert a metal object such as a knife into the cake and check if anything is sticking to the knife). It is a good idea to check on the cake after 30 minutes as ovens vary greatly in performance. The cake approximately doubles in size depending on the baking powder used.
(8) When the cake has cooled to room temperature cover it with a thin layer of melted chocolate. You have to be careful when melting the chocolate. First chop it up (100 g) then put it in a suitable container (eg beaker) and melt it using a hot water bath whilst stirring. Do NOT add water, milk or anything else. Simply use good quality dark chocolate with a high cocoa content and you are in business.
Thursday, August 16, 2007
Retraction - Azepinoazepine or Viologen?
 However, after the paper was accepted in Angewandte Chemie someone (probably Thomas Vaid, Washington University, US) was kind enough to point out that they had in fact made some viologens (2) (See Vaid's paper on viologens here). I guess that paper didn't last long on the CV. In Yamaguchi's defence I will say that it isn't entirely trivial to sort out the 13C NMR spectra of these compounds and when you are actually trying to make something specific you have a tendency to get carried away at times. I just hope this sort of thing never happens to me. On a similar note someone I know recently had a rather controversial opinion paper accepted in a high impact journal. However, whilst making changes suggested by the referees she came to the realisation that they couldn't actually draw the conclusions they had been making in the first place......I guess that's better than having to retract it later on but it's gotta hurt. D!
However, after the paper was accepted in Angewandte Chemie someone (probably Thomas Vaid, Washington University, US) was kind enough to point out that they had in fact made some viologens (2) (See Vaid's paper on viologens here). I guess that paper didn't last long on the CV. In Yamaguchi's defence I will say that it isn't entirely trivial to sort out the 13C NMR spectra of these compounds and when you are actually trying to make something specific you have a tendency to get carried away at times. I just hope this sort of thing never happens to me. On a similar note someone I know recently had a rather controversial opinion paper accepted in a high impact journal. However, whilst making changes suggested by the referees she came to the realisation that they couldn't actually draw the conclusions they had been making in the first place......I guess that's better than having to retract it later on but it's gotta hurt. D!Thursday, July 26, 2007
Behold cyclohexane!
 Recently I was flipping through a magazine published by a learned society and my eyes were caught by this rather interesting picture. The aim of this photo is to encourage scientist to publish in certain society journals. Firstly, it seems rather lame that the teachers is pointing at cyclohexane that appears to be in its perfectly flat conformation? Is that the most acidic proton he's pointing at? Secondly, what is going on with the students. I have a feeling the guy to the left is a werewolf. Or maybe he's just split up with the girl to the far right which would explain why she looks like she's about to commit murder. And what's going on with the woman in the background? It's cyclohexane people and not Brevetoxin so stop looking at it like that. D!
Recently I was flipping through a magazine published by a learned society and my eyes were caught by this rather interesting picture. The aim of this photo is to encourage scientist to publish in certain society journals. Firstly, it seems rather lame that the teachers is pointing at cyclohexane that appears to be in its perfectly flat conformation? Is that the most acidic proton he's pointing at? Secondly, what is going on with the students. I have a feeling the guy to the left is a werewolf. Or maybe he's just split up with the girl to the far right which would explain why she looks like she's about to commit murder. And what's going on with the woman in the background? It's cyclohexane people and not Brevetoxin so stop looking at it like that. D!Monday, July 23, 2007
Septanosides

Interesting work by Ganesh and Jayaraman at the Indian Institute of Science in Bangalore. D!
Monday, July 16, 2007
Oxepane Nucleic Acids - Part II
-
In the preivious post I gave the Tm data for homo-adenine and homo-thymine Oxepane Nucelic Acids (ONA) and it was quite clear that the low melting temperatures renders ONA useless from a pharmaceutical point of view. However, as I said it has a high stability in serum and activates RNase H. To date only very few oligonucleotide analogues have activated RNase H. The authors seem to be of the opinion that only four RNase H activating oligonucleotide (ON) analogues have been reported to date. However, they seem to forget the very first and most famous analogue - Phosphorus monothioates (PS). PS have been well known since the early 80s and ISIS Pharmaceuticals tried deveoloping drugs based on this class of analogues for a loooong time (they may still be doing so for all that I know). Anyway, let's have a look at the chemistry. They choose a somewhat surprising starting material and do a very funky Vorbrüggen coupling. I've never seen anything quite like it (notice the counterintuitive stereochemical outcome). Very impressive although the yields are poor.
 They get a fair bit of the diene oxepan product and some of the alfa-anomer too. Nevertheless a nice piece of work. Apparently an adaption of some work by Hoberg (JOC, 1997, 62, p. 6615) that I haven't checked out. People who haven't worked with nucleosides probably don't realise how difficult even the simplest transformations can be a times. Thymine and Adenine are by far the two "easiest" nucleobases to deal with. Cytosin is bad news and Guanine can be an outright nightmare. This is probably the main reason why everyone tests T and A first. Anyway, after taking the protection group off they proceed to hydrogenate the olefin which goes well. However, from here on it's a bit nasty. Firstly, they decide to use monomethoxy trityl (MMT) rather than dimethoxy trityl (DMT) because they get better yields this way. Now different molecules behave differently but I have a hard time accepting that you can't get DMT on in a higher yield than they report for MMT protection. Very odd! I wish they informed what happens instead.
They get a fair bit of the diene oxepan product and some of the alfa-anomer too. Nevertheless a nice piece of work. Apparently an adaption of some work by Hoberg (JOC, 1997, 62, p. 6615) that I haven't checked out. People who haven't worked with nucleosides probably don't realise how difficult even the simplest transformations can be a times. Thymine and Adenine are by far the two "easiest" nucleobases to deal with. Cytosin is bad news and Guanine can be an outright nightmare. This is probably the main reason why everyone tests T and A first. Anyway, after taking the protection group off they proceed to hydrogenate the olefin which goes well. However, from here on it's a bit nasty. Firstly, they decide to use monomethoxy trityl (MMT) rather than dimethoxy trityl (DMT) because they get better yields this way. Now different molecules behave differently but I have a hard time accepting that you can't get DMT on in a higher yield than they report for MMT protection. Very odd! I wish they informed what happens instead.  Maybe di-protection is a problem for some reason? Furthermore, they proceed to synthesise the phosphoramidite using the classic phosphoramidochlorodite (PCl) reagent. I see this all the time. Virtually, everyone in the area is using this phosphitylation reagent instead of the superior phosphordiamidite (PN2) reagent (See below).
Maybe di-protection is a problem for some reason? Furthermore, they proceed to synthesise the phosphoramidite using the classic phosphoramidochlorodite (PCl) reagent. I see this all the time. Virtually, everyone in the area is using this phosphitylation reagent instead of the superior phosphordiamidite (PN2) reagent (See below).  Back in the Jurassic when I used to make phosphoramidites I always used PN2. As a consequence no chromatography was necessary and I got >95% yield. We published a paper on how to make LNA (pioneered by Wengel) phosphoramidites using this reagent some years ago (Synthesis, 2002, 6, p. 802). Anyone interested in improving their phosphoramidite synthesis can request a copy (curlyarrow@gmail.com). So later on they proceed to synthesise their oligonucleotides and only
Back in the Jurassic when I used to make phosphoramidites I always used PN2. As a consequence no chromatography was necessary and I got >95% yield. We published a paper on how to make LNA (pioneered by Wengel) phosphoramidites using this reagent some years ago (Synthesis, 2002, 6, p. 802). Anyone interested in improving their phosphoramidite synthesis can request a copy (curlyarrow@gmail.com). So later on they proceed to synthesise their oligonucleotides and only  achieve coupling efficiencies of 98-99%. As always it is hard to know why the yield is reduced. I wonder if the use of MMT protection has anything to do with it. One final comment regarding their conclusion. They seem to spot a connection between RNase H activity of ON analogues and sugar conformer flexibility along the ON strand. However, they have just mentioned that alpha-L-LNA is a known ON analogue that activates RNase H. This analogue contains a highly constrained bicyclic sugar (See Figure) and hence doesn't support their conclusion. They could conceivably be right. Maybe some other mechanisms are at work with alpha-L-LNA but I think they should at least have mentioned this. Anyway, overall a good paper from this Canadian research group. Keep them coming. D!
achieve coupling efficiencies of 98-99%. As always it is hard to know why the yield is reduced. I wonder if the use of MMT protection has anything to do with it. One final comment regarding their conclusion. They seem to spot a connection between RNase H activity of ON analogues and sugar conformer flexibility along the ON strand. However, they have just mentioned that alpha-L-LNA is a known ON analogue that activates RNase H. This analogue contains a highly constrained bicyclic sugar (See Figure) and hence doesn't support their conclusion. They could conceivably be right. Maybe some other mechanisms are at work with alpha-L-LNA but I think they should at least have mentioned this. Anyway, overall a good paper from this Canadian research group. Keep them coming. D! Wednesday, July 11, 2007
Oxepane Nucleic Acids - Part I
 development targeting RNA rather than proteins. The idea is to knock the RNA out before it gets translated into protein (See figure). This is achieved by synthesising an antisense ON that is complementary to your RNA target. The mechanism of action for antisense is either:
development targeting RNA rather than proteins. The idea is to knock the RNA out before it gets translated into protein (See figure). This is achieved by synthesising an antisense ON that is complementary to your RNA target. The mechanism of action for antisense is either:-
(a) Inhibit the translation to protein by physically blocking the RNA strand making it impossible for ribosomes to translate it
or
(b) Activate the enzyme RNase H that specifically targets DNA-RNA duplexes and only degrades the RNA strand.
-
A lot of people in the field believe that antisense can only work effectively with RNase H activation and I tend to agree. The cell is amazingly efficient at making RNA and translating it to protein so if you have to get stoichiometric amounts of antisense ON to RNA into the cell you are likely to have a problem. The beauty with RNase H activation is that the system is catalytic. In other words the antisense ON gets released after RNA degradation and moves on to the next victim. The problem is that you cannot use regular DNA for antisense purposes as it has a very short half life in serum (~15 minutes). So you have to devise an analogue that is stable in serum, has high affinity towards RNA and activates RNase H. Now obviously this is no easy feat so why bother? The (theoretical) advantages when compared to traditional protein targeting drugs are:
-
(a) Complete selectivity only for the intended target
(b) You can target anything involving RNA
(c) The chemistry is the same every time. You just have to figure out what the sequence of your target is and synthesise the required ON
(d) Getting drugs to market is rapid because drug development is significantly faster
-
Obviously, things are much more complicated than this. Antisense was the big thing in the 80s. It was going to cure everything within the next decade but the reality is that only one product has made it to market. It's an ON called Vitravene (ISIS Pharmaceuticals) that prevents AIDS patients from going blind by targeting cytomegalovirus retinitis. That said a lot of advances have been made and there are numerous antisense ON in late stage clinical trials. Anyway, after this super condensed course in antisense ON I think we are ready for the actual paper. I'll let you off the hook for now. The next post should be up in a couple of days. D!
Friday, July 06, 2007
Still breathing
 I happened to look at Curly Arrow the other day...it's now been over a month since my last post! Not good, not good at all. Taitauwai even enquired about my well being. Well I'm still alive (sort of). My brain is slightly fried. I'm trying very hard to get some papers written whilst also attempting to set a new lab up, get my new projects going and phase new group members in and make their projects take off....yes I'm fairly busy. All my blogging time has effectively become paper writing time. Anyway, I have lots of things I would like to share at Curly Arrow and I'll try real hard to get some stuff posted. Whilst getting new gear for the lab I stumbled over an old box containing a virtually unused Vibro-Mischer - Das ideale rührwerk für Labor und Betrieb. Check out this nice poster I found in the box. That is one sexy model they picked to promote their products. If you click on the image you will get an enlarged version. D!
I happened to look at Curly Arrow the other day...it's now been over a month since my last post! Not good, not good at all. Taitauwai even enquired about my well being. Well I'm still alive (sort of). My brain is slightly fried. I'm trying very hard to get some papers written whilst also attempting to set a new lab up, get my new projects going and phase new group members in and make their projects take off....yes I'm fairly busy. All my blogging time has effectively become paper writing time. Anyway, I have lots of things I would like to share at Curly Arrow and I'll try real hard to get some stuff posted. Whilst getting new gear for the lab I stumbled over an old box containing a virtually unused Vibro-Mischer - Das ideale rührwerk für Labor und Betrieb. Check out this nice poster I found in the box. That is one sexy model they picked to promote their products. If you click on the image you will get an enlarged version. D!Wednesday, June 06, 2007
The Return of Dylan Stiles
 Just as we thought that Dylan Stiles, formerly the host of blog.tenderbuton.com, was a dead and buried blogger he returns. Check his post on how to make pure nepetalactone (aka Kitty Crack) in your kitchen out here. D!
Just as we thought that Dylan Stiles, formerly the host of blog.tenderbuton.com, was a dead and buried blogger he returns. Check his post on how to make pure nepetalactone (aka Kitty Crack) in your kitchen out here. D!Monday, June 04, 2007
Asymmetric synthesis of vinylcyclopropanes
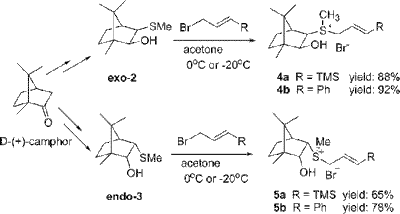 The work is very throrough and makes up an 11 page JACS paper (not including any experimental). Many chemists would probably have split this work up in two papers. It's really nice to see these guys decided to stick the whole story in one paper. In brief these guys discover that they can make trisubstituted vinyl-cyclopropanes in high yield, diastereoselectivity and enantioselectivity. Moreover, they can make both enantiomers of cyclopropane selectively by switching from endo- to exo-sulfur ylides. This table from the paper illustrates how sweet this stuff is:
The work is very throrough and makes up an 11 page JACS paper (not including any experimental). Many chemists would probably have split this work up in two papers. It's really nice to see these guys decided to stick the whole story in one paper. In brief these guys discover that they can make trisubstituted vinyl-cyclopropanes in high yield, diastereoselectivity and enantioselectivity. Moreover, they can make both enantiomers of cyclopropane selectively by switching from endo- to exo-sulfur ylides. This table from the paper illustrates how sweet this stuff is: Only "problem" here is that they are using stoichiometric sulfur ylide. However, they address this by developing a catalytic ylide cyclopropanation. The yields are not as impressive and the ee's are down to 50-80%. Still pretty cool and I bet these guys are working hard to improve the catalytic system. Finally, they decide to pull off a short and high yielding formal total synthesis of a known cyclopropane amino acid.
Only "problem" here is that they are using stoichiometric sulfur ylide. However, they address this by developing a catalytic ylide cyclopropanation. The yields are not as impressive and the ee's are down to 50-80%. Still pretty cool and I bet these guys are working hard to improve the catalytic system. Finally, they decide to pull off a short and high yielding formal total synthesis of a known cyclopropane amino acid. Obviously, they are making both enantiomers as well as both enantiomers of a diastereoisomer. And here I'm messing around trying to improve my lousy dr's on the racemic synthesis of the same target. Crap! D!
Obviously, they are making both enantiomers as well as both enantiomers of a diastereoisomer. And here I'm messing around trying to improve my lousy dr's on the racemic synthesis of the same target. Crap! D!Friday, June 01, 2007
You're fired
Tuesday, May 15, 2007
The Mannich Reaction revisited
 The Mannich Reaction (Carl Ulrich Franz Mannich, 1877-1947) is yet another one of those reactions that look brilliant on paper. However, I have on many occasions heard chemists attempting the reaction moan a fair bit to say the least. The major problem seems to be that the reaction is sluggish requiring heating/reflux to get anywhere and that the reagents (and desired product) start polymerising. You can find Mannich's original paper here: Mannich, C.; Krosche, W. Arch. Pharm. 1912, 250, p. 647. There is a detailed entry in Wikipedia on the reaction for those not familiar with it. A good alternative to the classic Mannich conditions is to use Eschenmoser's salt which I've seen used successfully in a number of total syntheses. Anyway, recently a PhD student in my lab was bitching about his Mannich Reaction. He left the lab, did some reading and came back with this nice JOC Note by A. Erkkila and P. M. Pihko, DOI: 10.1021/jo052529q. When he started using this stuff all his problems were solved. Fortunately, he sorted all this out right before I had to do my first Mannich Reaction. It also worked as a charm for me so I warmly recommend this simple, and efficient Mannich protocol.
The Mannich Reaction (Carl Ulrich Franz Mannich, 1877-1947) is yet another one of those reactions that look brilliant on paper. However, I have on many occasions heard chemists attempting the reaction moan a fair bit to say the least. The major problem seems to be that the reaction is sluggish requiring heating/reflux to get anywhere and that the reagents (and desired product) start polymerising. You can find Mannich's original paper here: Mannich, C.; Krosche, W. Arch. Pharm. 1912, 250, p. 647. There is a detailed entry in Wikipedia on the reaction for those not familiar with it. A good alternative to the classic Mannich conditions is to use Eschenmoser's salt which I've seen used successfully in a number of total syntheses. Anyway, recently a PhD student in my lab was bitching about his Mannich Reaction. He left the lab, did some reading and came back with this nice JOC Note by A. Erkkila and P. M. Pihko, DOI: 10.1021/jo052529q. When he started using this stuff all his problems were solved. Fortunately, he sorted all this out right before I had to do my first Mannich Reaction. It also worked as a charm for me so I warmly recommend this simple, and efficient Mannich protocol.
Now Erkkila and Pihko are quite concerned about reaction times because they are thinking of industry applications. However, for the average chemist that does a lot of work overnight (whilst at home in bed) it isn't essential that it's done in 1 hour. We found that if you do these reactions overnight no heating is required and the products are of very high purity. Very clean reactions indeed. Here's four examples from the paper:
 As it turns out the chemistry works really well for most systems using catalyst 1. However, some aldehydes require catalyst 2 to give a good result, eg. entries 3 and 4. The only compounds tested in this paper that failed completely were aldehydes that exist predominantly in a hemiacetal form, eg. 5-hydroxy-valeraldehyde. So there you have it. Maybe something you should consider giving a go next time it's alpha-methylenation time. D!
As it turns out the chemistry works really well for most systems using catalyst 1. However, some aldehydes require catalyst 2 to give a good result, eg. entries 3 and 4. The only compounds tested in this paper that failed completely were aldehydes that exist predominantly in a hemiacetal form, eg. 5-hydroxy-valeraldehyde. So there you have it. Maybe something you should consider giving a go next time it's alpha-methylenation time. D!
Sunday, May 13, 2007
Chemistry Blogs
 I've received some emails from people wanting me to link to their blogs and web sites and also had some people ask me why I don't provide links to certain chemistry blogs. The blogs on the lists are the ones I like and visit regularly. I have decided not to link to blogs were there is to much bitching and slander going on, in particular if the blogger is anonymous. And then there's obviously all the stuff that I still haven't had the time to check out. I've just added two new blogs this weekend that I would recommend. Organometallic Current is a great blog with detailed paper reviews and lots of mechanistic stuff. If you like your Palladium you should check it out. Also A Synthetic Environment is an excellent blog. This blog is looking at some more historic and equipment related topics which are quite entertaining. Check, check, cccheck it out man.... D!
I've received some emails from people wanting me to link to their blogs and web sites and also had some people ask me why I don't provide links to certain chemistry blogs. The blogs on the lists are the ones I like and visit regularly. I have decided not to link to blogs were there is to much bitching and slander going on, in particular if the blogger is anonymous. And then there's obviously all the stuff that I still haven't had the time to check out. I've just added two new blogs this weekend that I would recommend. Organometallic Current is a great blog with detailed paper reviews and lots of mechanistic stuff. If you like your Palladium you should check it out. Also A Synthetic Environment is an excellent blog. This blog is looking at some more historic and equipment related topics which are quite entertaining. Check, check, cccheck it out man.... D!Wednesday, May 09, 2007
Revenge of the NMR tube
 No my fingers weren't really blue. I have no idea why it keeps uploading the picture like this but it looks kind off cool and scientific. Anyway, it really wasn't particularly dramatic and if it wasn't for the glass I would never have gone to the Emergency Room. I have always heard that impaling yourself with an NMR tube is a particularly common accident amongst chemists. Nevertheless, I'm the first casualty that I know off. Does anyone else know of similar incidents? Finally, I have to say that there wasn't really anything I could have done to prevent this from happening. I used a brand new tube and applied a minimum amount of pressure when putting the lid on........just got unlucky I guess. And by the way what are people thinking off getting drunk and on drugs on a Monday. Save it for the weekend people. D!
No my fingers weren't really blue. I have no idea why it keeps uploading the picture like this but it looks kind off cool and scientific. Anyway, it really wasn't particularly dramatic and if it wasn't for the glass I would never have gone to the Emergency Room. I have always heard that impaling yourself with an NMR tube is a particularly common accident amongst chemists. Nevertheless, I'm the first casualty that I know off. Does anyone else know of similar incidents? Finally, I have to say that there wasn't really anything I could have done to prevent this from happening. I used a brand new tube and applied a minimum amount of pressure when putting the lid on........just got unlucky I guess. And by the way what are people thinking off getting drunk and on drugs on a Monday. Save it for the weekend people. D! Friday, May 04, 2007
Tethered aminohydroxylations Donohoe stylie
(1) Donohoe et al., Chem Comm, 2001, pp 2078-2079 (DOI: 10.1039/b107253f)
TA of acyclic, allylic carbamates using tert-butyl hypochlorite as the reoxidant with 4 mol% osmium. Yields ranging from 41 to 61%. Here's a really nice example with a diene:

(2) Donohoe et al., JACS, 2002, pp 12934-12935 (DOI: 10.1021/ja0276117)
TA of cyclic, allylic carbamates using tert-butyl hypochlorite as the reoxidant with 4 mol% osmium. Yields ranging from 50 to 83%. Works for 6,7 and 8-membered rings but only 5-membered rings with exocyclic double bonds undergo aminohydroxylation. Here's another nice example making a protected amino-sugar:

(3) Donohoe et al., Org. Lett., 2004, pp 2583-2585 (DOI: 10.1021/ol049136i)
TA of chiral acyclic, allylic carbamates using tert-butyl hypochlorite as the reoxidant with 4 mol% osmium. Yields ranging from 57 to 74% with excellent syn-selectivity. Some very impressive examples of TA reactions in this paper, for example:

(4) Donohoe et al., JACS, 2006, pp 2514-2515 (DOI: 10.1021/ja057389g)
 Nice stuff innit and it gets better.
Nice stuff innit and it gets better.
So it took about 6 years to develop this methodology to the point where I believe it will start finding wide spread use in synthesis. I'm itching to try one of these for myself and I'm desperately looking for an excuse. If anyone has tried running some of these Donohoe TAs I would very much like to hear any comments - is it really as good as it looks on paper? D!
Wednesday, May 02, 2007
Monkeys
 You'll notice that neither Europe nor North America has any monkeys. Where I used to work we saw many similarities between PhD students and monkeys (except monkeys have better lives). However, this is clearly not reflected on this map. Anyway, serious stuff tomorrow. D!
You'll notice that neither Europe nor North America has any monkeys. Where I used to work we saw many similarities between PhD students and monkeys (except monkeys have better lives). However, this is clearly not reflected on this map. Anyway, serious stuff tomorrow. D!
Friday, April 20, 2007
Tempo Oxidations Part II
 Fortunately, all the ingredients are reasonably affordable. Mixing it all up and adding the alcohol gives a biphasic reaction mixture that looks a bit like Schweppes Orange:
Fortunately, all the ingredients are reasonably affordable. Mixing it all up and adding the alcohol gives a biphasic reaction mixture that looks a bit like Schweppes Orange: However, unlike Delfourne et al. my final product wasn't clean after a simple work up. Succinimide was simply precipitating everywhere and hence some silica was required. In the end a filtration through a silica plug proved sufficient to give clean product on a reasonably large scale (18 grams) in excellent yield (97%). So despite the fact that a simple work up wasn't sufficient to clean the product up this is an easy to do reaction that I would recommend to anyone who's tired of stinky old Swern. D!
However, unlike Delfourne et al. my final product wasn't clean after a simple work up. Succinimide was simply precipitating everywhere and hence some silica was required. In the end a filtration through a silica plug proved sufficient to give clean product on a reasonably large scale (18 grams) in excellent yield (97%). So despite the fact that a simple work up wasn't sufficient to clean the product up this is an easy to do reaction that I would recommend to anyone who's tired of stinky old Swern. D! Monday, April 16, 2007
Fun with singlet oxygen
 Now the above reaction is obviously totally irresponsible and was only on for less than a minute so that I could take the picture. We are dealing with a fancy piece of glassware that has oxygen bubbling through it whilst two flood lamps are hammering photons away generating singlet oxygen and in the process heating the fume hood up big time. Hence, aluminium foil on the bottom of the hood is required to literally avoid a melt down and I'll be hooking a pump up that sends ice water through the cooling jacket. Moreover, I'm synthesising an endoperoxide - AAaARRRrGGGHhHh PEROXIDE - yeah I know but they are really quite stable. In fact our friends at the defence force haven't been able to blow them up so we aren't too worried. However, to avoid any nasty surprises we try not to make more than 5-10 grams of endoperoxides at any time. So as I said totally irresponsible hence I proceed to wrap this beautiful reaction up in two blasts shields covered in aluminum foil, pull the sash down and hook a cooling box/pump up to the glass ware and the final set up looks like this:
Now the above reaction is obviously totally irresponsible and was only on for less than a minute so that I could take the picture. We are dealing with a fancy piece of glassware that has oxygen bubbling through it whilst two flood lamps are hammering photons away generating singlet oxygen and in the process heating the fume hood up big time. Hence, aluminium foil on the bottom of the hood is required to literally avoid a melt down and I'll be hooking a pump up that sends ice water through the cooling jacket. Moreover, I'm synthesising an endoperoxide - AAaARRRrGGGHhHh PEROXIDE - yeah I know but they are really quite stable. In fact our friends at the defence force haven't been able to blow them up so we aren't too worried. However, to avoid any nasty surprises we try not to make more than 5-10 grams of endoperoxides at any time. So as I said totally irresponsible hence I proceed to wrap this beautiful reaction up in two blasts shields covered in aluminum foil, pull the sash down and hook a cooling box/pump up to the glass ware and the final set up looks like this: Well at least I know it's beautiful behind all that plastic and aluminium foil. This particular day I was doing the following photolysis:
Well at least I know it's beautiful behind all that plastic and aluminium foil. This particular day I was doing the following photolysis: 
Reactions of this type generally work quite well giving yields in the 40-70% range and since dienes are easily accessible using classic Wittig chemistry we consider making endoperoxides quite trivial. So why was I making this particular endoperoxide? Well if I told you I would have to kill you. There should be a paper coming out later this year featuring amongst others this particular endoperoxide turning into a supa cool cyclopropane in one (yes one) synthetic step so keep your eyes open for that paper. Some of you are probably wondering what Rose Bengal is. Behold the halogenated beast: We tend to use the bis-triethyl ammonium salt (as shown) because it is nicely soluble in organic solvents such as dichloromethane. D!
We tend to use the bis-triethyl ammonium salt (as shown) because it is nicely soluble in organic solvents such as dichloromethane. D!




 orcid.org/0000-0003-3926-7047
orcid.org/0000-0003-3926-7047


